How is electrolysis used in the industry?
How is electrolysis used in the industry?
Application of electrolysis in industry:
There are many industrial applications of electrolysis. The most common applications are as follows:
- Extraction of metals
- Purification of metals
- Electroplating of metals
Extraction of Metals Through Electrolysis
- Very reactive metals can only be extracted from their ores by electrolysis.
- Examples include the extraction of aluminium from molten aluminium oxide and sodium from molten sodium chloride.
Extraction of aluminium:
- Aluminium is extracted from its ore, bauxite, which contains aluminium oxide, Al2O3.
- Figure shows the electrolytic cell used for the extraction of aluminium.
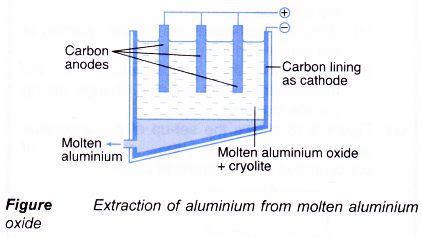
- Aluminium oxide is mixed with cryolite, Na3AlF6. Cryolite is added to lower the melting point of aluminium oxide (2045°C) to about 900°C.
- Blocks of carbon act as the anodes while the carbon lining of the cell acts as the cathode.
- At the cathode, the aluminium ions are discharged to form aluminium metal.
Al3+(l) + 3e– → Al(l)
Liquid aluminium is denser than the electrolyte and will be collected at the bottom of the cell. - At the anode, the oxide ions are discharged to form oxygen gas.
2O2-(l) → O2(g) + 4e– - The overall chemical reaction is:
2Al2O3(l) → 4Al(l) + 3O2(g) - The oxygen liberated at the anode will react with the carbon electrode to produce carbon dioxide gas.
C(s) + O2(g) → CO2(g)
Consequently, the anode is corroded slowly and must be replaced from time to time.
Extraction of sodium:
- Sodium is extracted from molten sodium chloride, NaCl.
- Figure shows the electrolytic cell used for the extraction of sodium.
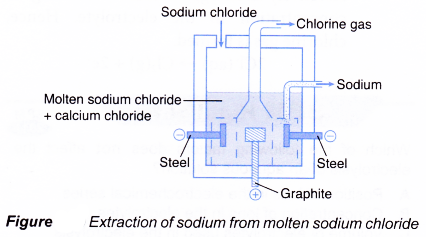
- Sodium chloride is mixed with calcium chloride, CaCl2. Calcium chloride is added to lower the melting point of sodium chloride (900°C) to about 600°C.
- The cathode is made of steel while the anode is made of graphite.
- At the cathode, the sodium ions are discharged to form sodium metal.
Na+(l) + e– → Na(l) - At the anode, the chloride ions are discharged to form chlorine gas.
2Cl– (l) → Cl2(g) + 2e– - The overall chemical reaction is:
2NaCl(l) → 2Na(l) + Cl2(g)
People also ask
- Why is an electrolyte able to conduct electricity while a Nonelectrolyte Cannot?
- Analysing the electrolysis of molten compounds
- Analysing the Electrolysis of Aqueous Solutions
- What does electrochemical series mean?
- How does a voltaic cell work?
Purification of Copper by Electrolysis
- Metals extracted from their ores may contain other metals as impurities. Electrolysis can be used to purify these metals.
- In the purification of metals, the impure metal is used as the anode and the pure metal is used as the cathode.
- The electrolyte used is an aqueous salt solution of the metal.
To purify a metal,
- the impure metal is made the anode.
- the pure metal is made the cathode.
- the electrolyte is a solution of the metal ions.
Purification of Copper by Electrolysis Experiment
Aim: To study the purification of copper.
Problem statement: How can the electrolysis process be used to purify copper?
Hypothesis: When impure copper is used as the anode and pure copper is used as the cathode during the electrolysis of copper(II) sulphate solution, the impure copper can be purified.
Variables:
(a) Manipulated variable : Positions of the pure copper and impure copper as the electrodes
(b) Responding variable : Deposition of copper on the pure copper plate
(c) Controlled variables : Type of electrolyte, concentration of electrolyte, duration of electrolysis
Materials: 1 mol dm-3 copper(II) sulphate solution, impure copper plate and pure copper plate.
Apparatus: Batteries, 250 cm3 beaker, connecting wires with crocodile clips, ammeter and switch.
Procedure:
- A beaker is filled with 1 mol dm-3 copper(II) sulphate solution until it is half full.
- The apparatus is set up as shown in Figure, using the impure copper plate as the anode and the pure copper plate as the cathode.
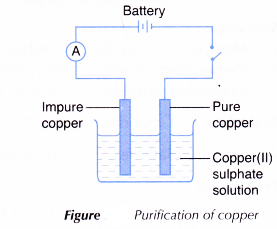
- The switch is turned on to allow the electric current to pass through the electrolyte for 40 minutes.
- The changes at the anode and the cathode are recorded.
- Steps 1 to 4 are repeated using impure copper as the cathode and pure copper as the anode.
Observations:
| Electrode | Observation | ||
| Anode | Cathode | Anode | Cathode |
| Impure copper | Pure copper | The impure copper electrode dissolves into the solution. The anode becomes thinner. | The pure copper electrode becomes thicker. |
| Pure copper | Impure copper | The pure copper electrode dissolves into the solution. The anode becomes thinner. | The impure copper electrode becomes thicker. |
Discussion:
- During the electrolysis using impure copper as the anode and pure copper as the cathode, the impure copper plate dissolves and the impurities fall to the bottom of the beaker.
Cu(s) → Cu2+(aq) + 2e–
The copper(II) ions are discharged at the pure copper plate. A layer of copper is deposited on the pure copper plate.
Cu2+(aq) + 2e– → Cu(s)
Purification of copper occurs. - During the electrolysis using pure copper as the anode and impure copper as the cathode, the pure copper plate dissolves while copper is deposited on the impure copper plate. Purification of copper does not occur.
Conclusion:
Purification of copper occurs when the anode is impure copper and the cathode is pure copper. The electrolyte is copper(II) sulphate solution. The hypothesis is accepted.
Electroplating of Metals Using Electrolysis
- Electroplating is a process of depositing a layer of metal on another substance using electrolysis.
- Objects are electroplated to protect them from corrosion and to give them an attractive appearance.
- To electroplate an object with a metal,
- the object to be plated must become the cathode.
- the anode would be the pure plating metal.
- the electrolyte should contain ions of the plating metal.
- The conditions that must be satisfied for good quality plating are as follows:
- The metal object to be plated must be clean and free of grease.
- The concentration of the ions of the plating metal must be low.
- The electric current must be small.
- The object to be plated must be turned steadily.
The effects of the use of electrolysis in industries
The benefits of electrolysis in industries are as follows:
- Reactive metals can be extracted by electrolysis.
- A very thin layer of metal can be coated on an object using electrolysis.
- Electrolysis can be used, to produce a very pure metal. For example, copper can be purified up to 99.98% by electrolysis.
The disadvantages of electrolysis are as follows.
- Electrolysis may cause pollution.
- Electrolysis is an expensive process because it requires a large amount of energy.
Electroplating of Metals Using Electrolysis Experiment
Aim: To study the electroplating of an iron spoon with copper.
Problem statement: How can an iron spoon be coated with copper through electrolysis?
Hypothesis: When an iron spoon is used as the cathode and copper metal is used as the anode during the electrolysis of copper(II) sulphate solution, the iron spoon can be coated with copper.
Variables:
(a) Manipulated variable : Position of the iron spoon as an electrode
(b) Responding variable : Deposition of copper on the iron spoon
(c) Controlled variables : Type of electrolyte, concentration of electrolyte, duration of electrolysis
Materials: 1 mol dm-3 copper(II) sulphate solution, copper electrode, iron spoon and sandpaper.
Apparatus: Batteries, 250 cm3 beaker, connecting wires with crocodile clips, ammeter, switch and rheostat.
Procedure:
- An iron spoon is cleaned with sandpaper. The iron spoon is washed with detergent and it is then rinsed thoroughly with water.
- Two-thirds of a beaker is filled with copper(II) sulphate solution.
- The apparatus is set up using the iron spoon as the cathode and a copper electrode as the anode as shown in Figure.
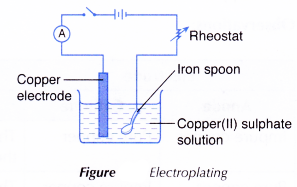
- The switch is turned on and the current is adjusted to 0.2 A using the rheostat.
- The current is turned off after 30 minutes. Figure Electroplating
- The iron spoon is removed from-the electrolyte and it is dried. The change to the iron spoon is then recorded.
- Steps 1 to 6 are repeated using copper as the cathode and the iron spoon as the anode.
Observations:
| Anode | Cathode | Observation |
| Copper | Iron spoon | A brown solid is deposited on the iron spoon. The copper electrode becomes thinner. |
| Iron spoon | Copper | No brown solid is deposited on the iron spoon. The copper electrode becomes thicker. |
Discussion:
- During the electrolysis using copper as the anode and an iron spoon as the cathode, the copper anode dissolves to form copper(II) ions.
Cu(s) → Cu2+(aq) + 2e–
At the cathode, copper metal is deposited on the surface of the iron spoon.
Cu2+(aq) + 2e– → Cu(s)
Electroplating of the iron spoon with copper occurs. - During the electrolysis using an iron spoon as the anode and copper as the cathode, the anode dissolves while copper is deposited on the copper cathode. Electroplating of the iron spoon with copper does not occur.
Conclusion:
Electroplating of an iron spoon with copper occurs when the cathode is the iron spoon and the anode is the copper. The electrolyte is copper(II) sulphate solution. The hypothesis is accepted.
The post How is electrolysis used in the industry? appeared first on A Plus Topper.
from A Plus Topper
via Learning Made Simple 360
*Note that these contents are Autoblogged from A Plus Topper and cannot be edited.
Join the conversation