What is the periodic table of the elements?
What is the periodic table of the elements?
What is the history of the periodic table?
The scientists involved in the development of the Periodic Table were:
Antoine Lavoisier, Johann W. Dobereiner, John Newlands, Lothar Meyer, Dmitri Mendeleev and H.J.G. Moseley
Contribution by Antoine Lavoisier (1743 – 1794)
- Antoine Lavoisier, a French chemist, was the first person to classify elements into groups.
- In the year 1789, the known elements at that time were classified into four groups as shown in Table.
Group 1 Group 2 Group 3 Group 4 Oxygen
Nitrogen
Hydrogen
Light
HeatSulphur
Phosphorus
Carbon
Chlorine
FluorineArsenic
Bismuth
Cobalt
Lead
Zinc
Nickel
Tin
SilverLime
Silica
Alumina
Barita
Magnesia - Antonie Lavoisier grouped the element into four categories based on their chemical properties: gases, non-metals, metals and earth.
- The classification by Antoine Lavoisier was unsucessful because his table consisted of many wrong information.
For example:
Light, heat and a few compounds that were unable to decompose into elements such as lime, silica, alumina, barita and magnesia were considered as elements in his table.
Contribution by Johann W. Dobereiner (1780 – 1849)
- In the year 1829, Johann W. Dobereiner, a German chemist, classified the elements into triads.
- Each triad consisted of three elements with similar chemical properties.
- In each triad, the relative atomic mass of the middle element was approximately the average relative atomic mass of the other two elements.
Table illustrates two examples of triads.
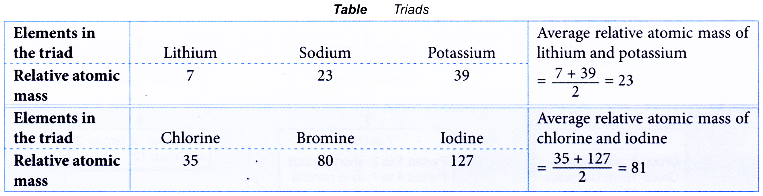
- Classification of the elements into triads by Dobereiner was unsuccessful because this classification was limited to a few elements only.
- However, the Triad Law had led scientists to realise that there was a relationship between the properties and the atomic masses of the elements.
People also ask
- Physical and Chemical Properties of Group 1 Elements
- Physical and Chemical Properties of Group 18 Elements
- Physical and Chemical Properties of Group 17 Elements
- What do you mean by transition metals?
- What is the Need for Classification of Elements?
- Modern Periodic Table and Its Significance
- How did Mendeleev Arrange the Periodic Table?
- Periodic Trends in Properties of Elements
- What is Valency and Atomic Radius?
- What are Metallic and Nonmetallic Properties?
Contribution by John Newlands (1837 – 1898)
- From the year 1864 to 1865, John Newlands, a British chemist, arranged all the known elements horizontally in ascending order of their atomic masses.
- Each row consisted of seven elements, as shown in Table.
1 2 3 4 5 6 7 H Li Be B C N O F Na Mg Al Si P S Cl K Ca Cr Ti Mn Fe Co,
NiCu Zn Y In As Se Br Rb Sr Ce,
LaZr Bi,
MoRh,
RuPd Ag Cd U Sn Sb Te - In his arrangement, the same properties were repeated at every eighth element. This pattern was similar to an octave of notes in music. This arrangement of elements was known as the law of octaves.
- The classification of elements by John Newlands was not successful because his law of octaves was obeyed by the first 17 elements only (from H to Ca).
- However, he made an important contribution as he was the first chemist to show the existence of a periodic pattern in the properties of the elements.
- The periodic repetition of the properties of the elements was used as a basis for further development of the Periodic Table.
Contribution by Lothar Meyer (1830 – 1895)
- In the year 1870, Lothar Meyer, a German chemist, plotted a graph of the atomic volume against the atomic mass for all the known elements at that time.
(Definition: Atomic volume of an element is the volume of one mole of atoms of that element.) - The curve obtained by Lothar Meyer is shown in Figure.
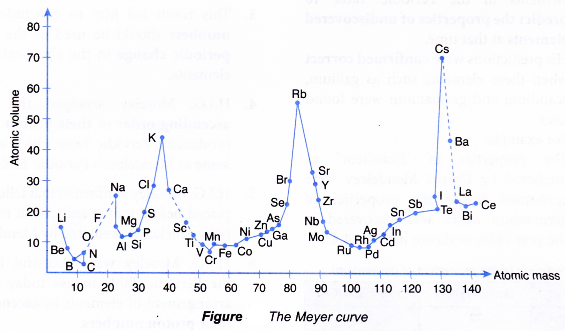
- Lothar Meyer realised that elements with similar chemical properties occupied the same relative positions on the curve.
- For example:
Li, Na, K, Rb and Cs (alkali metals) located at the peaks of the curve had similar chemical properties.
F, Cl, Br and I (halogens) located at the slopes of the curve also have similar chemical properties. - Based on these findings, Lothar Meyer compiled a Periodic Table of 56 elements in ascending order of atomic masses based on the periodicity of properties such as atomic volume.
- Lothar Meyer was successful in showing that the properties of the elements were in a periodic pattern with their atomic masses.
Contribution by Dmitri Mendeleev (1834 – 1907)
In the year 1869, Dmitri Mendeleev, a Russian chemistry professor, arranged the elements in ascending order of their atomic masses as carried out by John Newlands but made a few changes as described below:
- Elements with similar chemical properties were placed in the same vertical column called a group.
- Gaps were left in the Periodic Table for undiscovered elements at that time.
- He made use of the positions of the elements in the Periodic Table to predict the properties of undiscovered elements at that time.
- His predictions were confirmed correct when these elements such as gallium, scandium and germanium were found later.
- For example:
The properties of ‘Ekasilicon’ as predicted by Dmitri Mendeleev were confirmed to be the properties of germanium which was discovered in the year 1886, as shown in Table.Properties Ekasilicon (Es) Germanium (Ge) Atomic mass 72 72.6 Colour of metal Grey Grey Density of element 5.5 g cm-3 5.47 g cm-3 Density and formula of oxide 4.7 g cm-3, EsO2 4.7 g cm-3, GeO2 - He mutually exchanged the positions of nickel (atomic mass = 58.7) with cobalt (atomic mass = 58.9) and iodine (atomic mass = 126.9) with tellurium (atomic mass = 127.6) so that the elements with similar chemical properties were placed under the same group.
- He arranged certain elements such as manganese, iron, cobalt, nickel, copper and others in separate groups. These group of elements were called transition elements.
Dmitri Mendeleev was more successful compared to the other scientists in the development of the Periodic Table.
Contribution by H.J.G. Moseley (1887- 1915)
- In the year 1914, H.J.G. Moseley, a British physicist, carried out an experiment to measure the frequency of the X-ray released from different elements when these elements were bombarded by high energy electrons.
- He then plotted a graph of the square root of the frequency of X-ray emitted from the elements against their proton numbers. A straight line was obtained.
- This result led him to conclude that proton numbers should be used as the basis for the periodic change in the chemical properties of elements.
- H.J.G. Moseley arranged the elements in ascending order of their proton numbers. He produced a Periodic Table which was almost the same as Mendeleev s Periodic Table.
- H.J.G. Moseley confirmed that tellurium must be placed before iodine and cobalt must be placed before nickel as predicted by Mendeleev.
- H.J.G. Moseley was successful in developing the Periodic Table in use today based on the arrangement of elements in ascending order of their proton numbers.
How is the periodic table of the elements arranged?
Arrangement of elements in the Periodic Table
Figure shows the Periodic Table in use today.
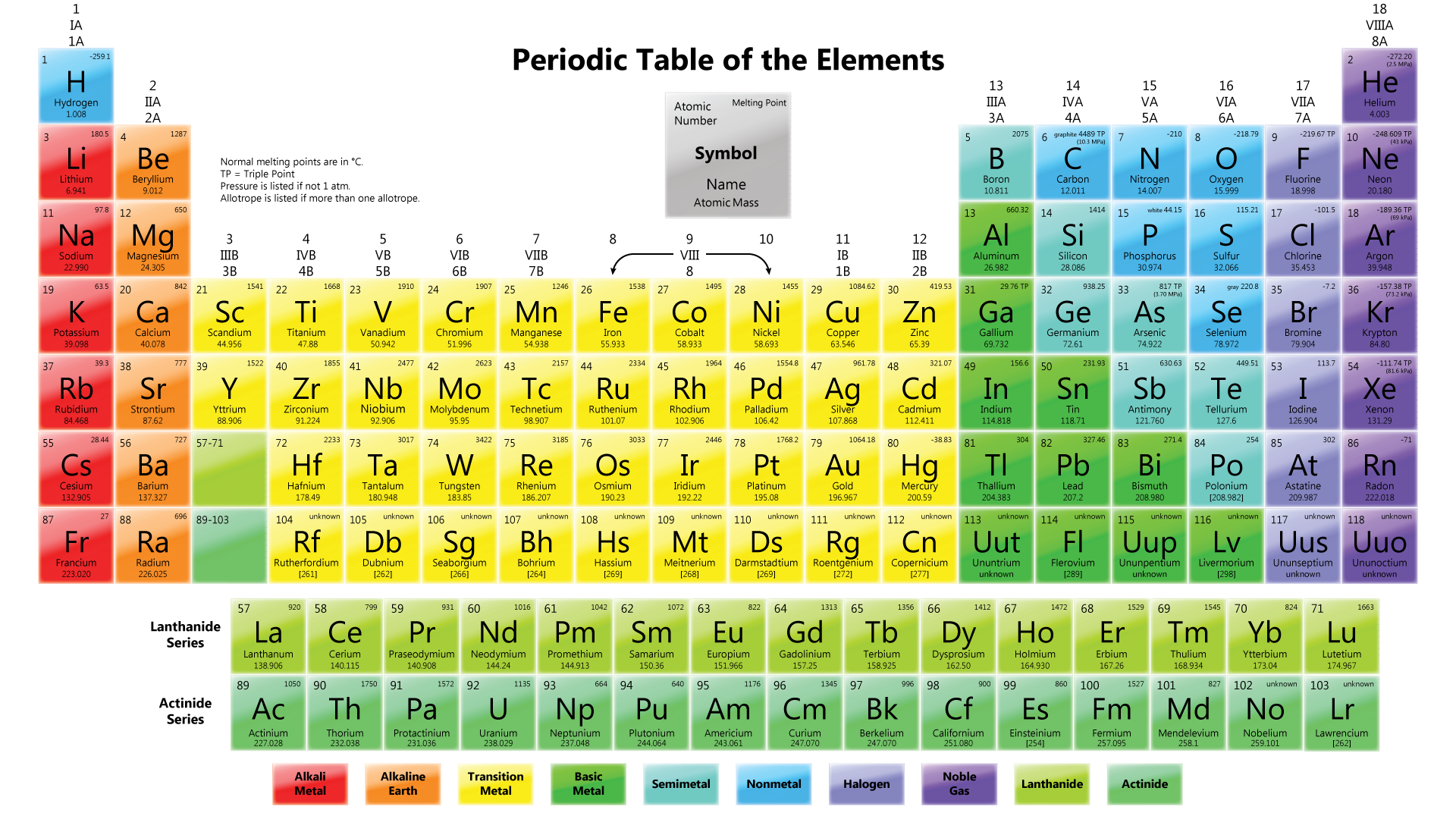 Elements are arranged horizontally In ascending order of their proton numbers, from 1 to 116, in the Periodic Table.
Elements are arranged horizontally In ascending order of their proton numbers, from 1 to 116, in the Periodic Table.
Groups
- Definition: Each vertical column of elements in the Periodic Table is known as a group.
- Elements with the same number of valence electrons are arranged in the same group.
- There are 18 vertical columns of elements in the Periodic Table known as Group 1, Group 2, until Group 18.
- Group 1 elements are known as alkali metals.
- Group 2 elements are known as alkaline earth metals.
- Group 3 to Group 12 elements are known as transition elements.
- Group 17 elements are known as halogens.
- Group 18 elements are known as noble gases.
Periods
- Definition: Each horizontal row of elements in the Periodic Table is known as a period.
- There are 7 horizontal rows of elements in the Periodic Table, known as Period 1, Period 2, until Period 7.
- Periods 1 to 3 are short periods while Periods 4 to 7 are long periods.
- Period 1 contains 2 elements.
- Periods 2 and 3 contain 8 elements respectively.
- Periods 4 and 5 contain 18 elements respectively.
- Period 6 contains 32 elements.
- Period 7 contains 27 elements.
- Although Period 6 contains 32 elements, elements with proton numbers 57 to 71 are arranged separately at the bottom of the Periodic Table. This series of elements is called lanthanides.
- Similarly, elements with proton numbers 89 to 103 in Period 7 are arranged separately at the bottom of the Periodic Table. This series of elements is called actinides.
Metallic and non-metallic properties
- Element in Group 1, 2 and 13 are metals.
- Transition elements in Group 3 to 12 are also metals.
- Elements in Group 15, 16, 17 and 18 are non-metals.
- In Group 14,
- Carbon and silicon are non-metals.
- Germanium is a metalloid (semimetal)
- Tin and lead are metals.
1. Relationship between the electron arrangement and the position of the element in the Periodic Table
Figure shows the electron arrangements of the elements with proton numbers 1 to 20 in the Periodic Table.
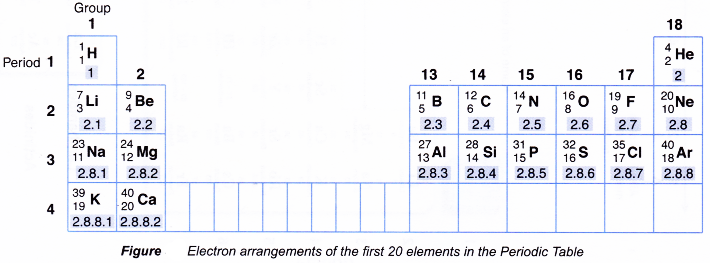
2. Relationship between the electron arrangement and the group number of an element
- Based on above Figure, the group number of an element is determined by the number of valence electrons in an atom of the element.
- Table shows the relationship between the number of valence electrons and the group number of an element.

- For elements with 1 or 2 valence electrons,
Group number of that element = Number of valence electrons - For elements with 3 to 8 valence electrons,
Group number of that element = Number of valence electrons plus 10
Note:
Helium with an electron arrangement of 2 is placed in Group 18. This is an exception.
This is because helium has similar inert properties as the other noble gases in Group 18.
Example: Element Q has a nucleon number of 27. An atom of element Q contains 14 neutrons. In which group is element Q located in the Periodic Table?
Solution:
Number of electrons in an atom Q = Number of protons
= 27 – 14 = 13
Electron arrangement of atom Q = 2.8.3
Number of valence electrons = 3
∴ Group number = 3 + 10 = 13
Hence, element Q is located in Group 13 of the Periodic Table.
3. Relationship between the electron arrangement and the period number of an element
- Based on above Figure, the period number of an element is determined by the number of shells occupied with electrons in an atom of that element.
- Table shows the relationship between the number of shells occupied with electrons and the period number of an element.

- Hence,
Period number of an element = Number of shells occupied with electrons in an atom of that element
Example: Element T has a proton number of 19 and a nucleon number of 39. In which period is element T located in the Periodic Table?
Solution:
Number of electrons in atom T
= Number of protons in atom T
= Proton number =19
∴ Electron arrangement of atom T = 2.8.8.1
Atom T has 4 shells occupied with electrons. Hence, element T is located in Period 4 of the Periodic Table.
Example: Element R is located in Group 15 and Period 3 of the Periodic Table. What is the electron arrangement of an atom of element R?
Solution:
Atom R has 5 valence electrons because it is in Group 15.
Atom R has 3 shells occupied with electrons because it is in Period 3.
Electron arrangement of atom R = 2.8.5
4. Elements with the same number of valence electrons will exhibit similar chemical properties.
For example:
Atom W with an electron arrangement of 2.8.2 and atom X with an electron arrangement of 2.8.8.2 exhibit similar chemical properties.
This is because both the atoms of W and X have 2 valence electrons, that is the same number of valence electrons.
The post What is the periodic table of the elements? appeared first on A Plus Topper.
from A Plus Topper
via Learning Made Simple 360
*Note that these contents are Autoblogged from A Plus Topper and cannot be edited.
Join the conversation