How can we separate a mixture of two miscible liquids
How can we separate a mixture of two miscible liquids
Separation of mixture of two or more liquid
All the mixtures containing two (or more) liquids can be separated by the following two methods:
- By the process of fractional distillation.
- By using a separating funnel.
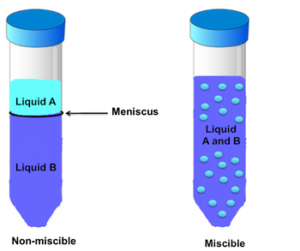
1. Miscible liquids :
Those liquids which mix together in all proportions and form a single layer are called miscible liquids.
Ex. Alcohol and water are miscible liquids because they mix together in all proportions and form a single layer on mixing. A mixture of miscible liquids is separated by the process of fractional distillation.
2. Immiscible liquids :
Those liquids which do not mix with each other and form separate layers are called immiscible liquids.
Ex. Oil and water are immiscible liquids because they do not mix with each other., and form separate layers on mixing. Water being heavier forms the lower layer, and oil being lighter forms the upper layer. A mixture of immiscible liquids is separated by using an apparatus called separating funnel.
 (i) Separation by fractional distillation :
(i) Separation by fractional distillation :
Fractional distillation is the process of separating two (or more) miscible liquids by distillation, the distillate being collected in fractions boiling at different temperatures. A mixture of two miscible liquids can be separated by the process of fractional distillation. The separation of two liquids by fractional distillation depends on the difference in their boiling points. Fractional distillation is carried out by using a fractionating column.
Ex. Alcohol and water are miscible liquids. The boiling point of alcohol is 78ºC and the boiling point of water is 100ºC. Since the boiling points of alcohol and water different, therefore, a mixture of alcohol and water can be separated by fractional distillation. The mixture of alcohol and water is heated in a distillation flask fitted with a fractionating column. When the mixture is heated, both alcohol and water form vapours as their boiling points approach. The alcohol vapour and water vapour rise up in the fractionating column. The upper part of the fractionating column is cooler, so as the hot vapours rise up in the column, they get cooled, condense and trickle back into the distillation flask.
 The more volatile liquid distils over first, and the less volatile liquid distils over later. A mixture of alcohol and water can be separated by fractional distillation.
The more volatile liquid distils over first, and the less volatile liquid distils over later. A mixture of alcohol and water can be separated by fractional distillation.
(ii) Separation by a separating funnel :
A mixture of two immiscible liquids can be separated by using a separating funnel. A separating funnel is a special type of funnel which has a stop-cock in its stem to allow the flow of a liquid from it, or to stop the flow of liquid from it. The separation of two immiscible liquids by a separating funnel depends on the difference in their densities.
The mixture of two immiscible liquids is put in a separating funnel and allowed to stand for some time. The mixture separates into two layers according to the densities of the liquids in it. The heavier liquid or denser liquid forms the lower layer whereas the lighter liquid forms the upper layer. On opening the stop-cock of separating funnel, the lower layer of heavier liquid comes out first and collected in a beaker. When the lower layer of heavier liquid has completely run off, the stop-cock is closed. The lighter liquid in the upper layer is collected in a separate beaker by opening the stop-cock again.
Ex. Water and kerosene oil are two immiscible liquids. So, a mixture of water and kerosene can be separated by using a separating funnel.
The post How can we separate a mixture of two miscible liquids appeared first on A Plus Topper.
from A Plus Topper
via Learning Made Simple 360
*Note that these contents are Autoblogged from A Plus Topper and cannot be edited.
Join the conversation