Properties and Uses of Ethanoic Acid
Properties and Uses of Ethanoic Acid

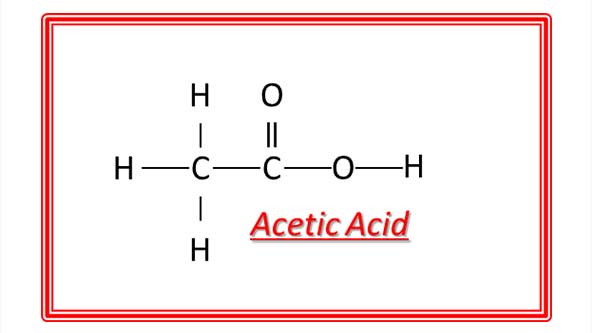

Ethanoic acid (Acetic acid) CH3COOH : Ethanoic acid is most commonly known as acetic acid. Its dilute solution in water (5-8%) is known as vinegar, which is used for preserving food-sausage, pickles etc.
Physical properties :
(i) Ethanoic acid is vinegar smelling liquid. The lower carboxylic acids are liquids whereas higher ones are solids.
(ii) Ethanoic acid is sour in taste. Other lower carboxylic acids are also sour in taste.
(iii) Ethanoic acid has boiling point 391 K. Carboxylic acids have higher boiling points than corresponding alcohols, aldehydes and ketones.
(iv) Acetic acid is soluble in water, i.e., it is miscible with water in all proportions. The lower carboxylic acids are soluble in water but solubility in water decreases with increase in molecular weight.
(v) Acetic acid freezes at 290 K. Thus, in cold weather crystallization of acetic acid may take place that is why pure acetic acid is called glacial acetic acid.
Chemical Properties :
(i) Ethanoic acid is weak acid but it turns blue litmus red.
(ii) Reaction with Metal. Ethanoic acid reacts with metals like Na, K, Zn etc. to form metal ethanoates and hydrogen gas.
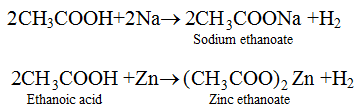
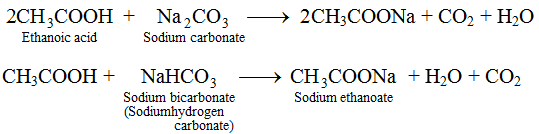
(iii) Reaction with Carbonates. Ethanoic acid reacts with bicarbonates and carbonates and produces brisk effervescence due to formation of carbon dioxide.
(iv) Reaction with Base. Ethanoic acid reacts with sodium hydroxide to form sodium ethanoate and water.
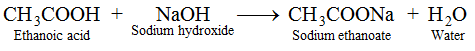
(v) Decarboxylation (Removal of CO2). When sodium salt of ethanoic acid, i.e., sodium ethanoate is heated with soda lime (3 parts of NaOH and 1 part of CaO), methane gas is formed.
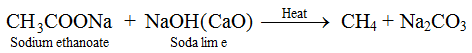
This reaction is known as decarboxylation because a molecule of CO2 is removed from a molecule of acid.
(vi) Reaction with alcohols. Ethanoic acid reacts with ethanol in presence of concentrated sulphuric acid to form esters which are pleasant fruity smelling compounds.
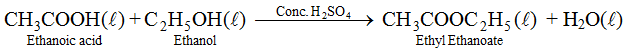
(vii) Reduction. Acetic acid, on reduction with lithium aluminium hydride, results in formation of ethanal, which on further reduction gives ethanol.
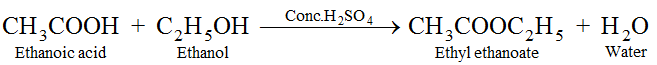
Uses of Ethanoic acid :
(i) It is used for making vinegar
(ii) It is used as a laboratory reagent
(iii) It is used for preparation of white lead [2PbCO3.Pb(OH)2] which is used in white paints.
(iv) It is used for coagulation of rubber from latex and casein (protein) from milk
(v) It is used in preparation of acetone, ethyl acetate, acetic anhydride, aspirin which is used in medicines.
(vi) It is used in preparation of cellulose acetate which is used for making photographic film.
(vii) Its esters are used in artificial flavours in perfumes.
(viii) Its 5% solution is bactericidal (destroys bacteria)
(ix) Its compound basic copper acetate (verdigris) is used as green pigment.
(x) Aluminium acetate and chromium acetate are used as mordants in dyeing and waterproofing of fabrics.
People also ask
- What are carbon compounds?
- Chemical Properties of Carbon Compounds
- How are alkanes formed?
- What is an alkene in chemistry?
- What is an isomerism?
- What is alcohol and how is it made?
- How are carboxylic acids formed?
- How esters are formed?
- What are fats and oils?
- How palm oil is extracted?
- Order in Homologous Series
- What is the monomer of natural rubber?
- Which acid is used for coagulating rubber from latex?
- Classification of Hydrocarbons
- What is the homologous series of hydrocarbons?
- Properties and Uses of Ethanol
The post Properties and Uses of Ethanoic Acid appeared first on A Plus Topper.
from A Plus Topper
via Learning Made Simple 360
*Note that these contents are Autoblogged from A Plus Topper and cannot be edited.
Join the conversation