What is an isomerism?
What is an isomerism?
What is an isomer and example?
Isomerism
- Isomerism is a phenomenon whereby two or more molecules are found to have the same molecular formula.
- These molecules have the same numbers and types of atoms. They only differ in the arrangement of the atoms. They are molecules which have different structures. These molecules are called isomers.
- Isomers are molecules with the same molecular formula, but with different structural formulae.
- Isomers have different physical properties.
- Isomers from the same homologous series have the same functional group. Hence, they have the same chemical properties.
How to draw isomers of alkanes?
Isomerism in alkanes
- Isomerism in alkanes starts with molecules with more than three carbon atoms. Methane, ethane and propane do not have isomers.
- Alkane isomers only differ in the arrangement of the atoms, producing straight-chain and branched-chain molecules.
For example, butane, C4H10 has two isomers shown below.
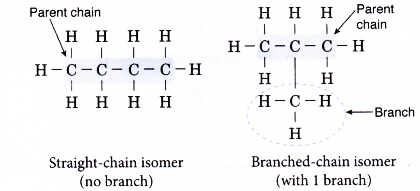
Use the following steps to help you draw structural formulae of isomers of alkanes.
- Step 1: Use the total number of carbon atoms given in the molecular formula to draw all the possible straight-chain and branched-chain carbon skeletons. This is done by varying the number of carbon atoms in the straight and branched chains.
- Step 2: For each carbon skeleton, place single bonds around each carbon atom to ensure each carbon atom has four bonds.
- Step 3: Complete the structure by placing one hydrogen atom at each of the single bonds.
Isomerism in alkanes example:
Draw all the isomers of C5H12.
Solution:
- Step 1: Draw all the possible carbon skeletons.
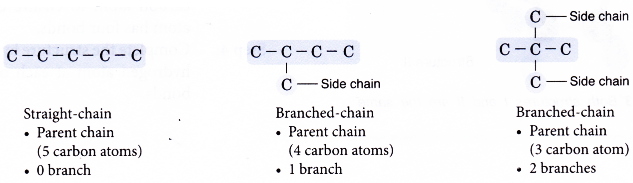
- Step 2: Place single bonds around every carbon atom. Ensure that each carbon atom has four bonds.
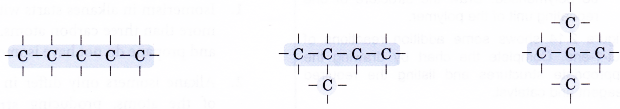
- Step 3: Place a hydrogen atom at each of the single bonds.
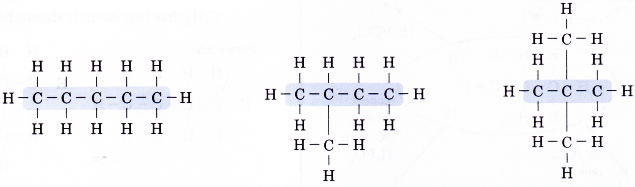
People also ask
- What are carbon compounds?
- Chemical Properties of Carbon Compounds
- How are alkanes formed?
- What is an alkene in chemistry?
- What is alcohol and how is it made?
- How are carboxylic acids formed?
- How esters are formed?
- What are fats and oils?
- How palm oil is extracted?
- Order in Homologous Series
- What is the monomer of natural rubber?
- Which acid is used for coagulating rubber from latex?
- Classification of Hydrocarbons
- What is the homologous series of hydrocarbons?
- Properties and Uses of Ethanol
- Properties and Uses of Ethanoic Acid
How to draw isomers of alkenes?
Isomerism in alkenes
- Alkenes also show isomerism. However, isomerism in alkenes is due to
(a) the branching of the carbon chain
(b) the different positioning of the double bond - Ethene and propene do not exhibit isomerism.
- Isomerism in alkenes starts with molecules with more than three carbon atoms. For example, butene, C4H8 has three isomers and pentene, C5H10 has five isomers.
Use the following steps to draw structural formulae of isomers of alkenes.
- Step 1: Use the total number of carbon atoms given in the molecular formula to draw all the possible straight-chain and branched-chain carbon skeletons. This is done by varying the number of carbon atoms in the straight and branched chains.
- Step 2: For each carbon skeleton, place a double bond at different locations.
- Step 3: Place single bonds around each carbon atom to ensure each carbon atom has four bonds.
- Step 4: Complete the structure by placing one hydrogen atom at each of the single bonds.
Isomerism in alkanes example:
Draw all the isomers of C4H8.
Solution:
- Step 1: Draw all the possible carbon skeletons.
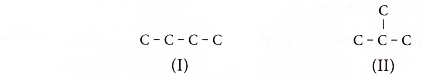
- Step 2: For each carbon skeleton, place a double bond at different locations.
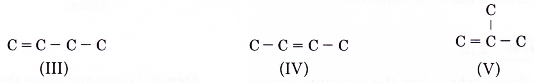
- Step 3: Place single bonds around each carbon atom. Ensure that each carbon atom has four bonds.
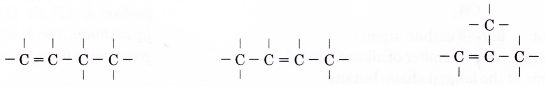
Note:
Placing the double bond between the last two carbon atoms in skeleton (I) produces the following structure.

But this structure is the same as skeleton (III).
Drawing a double bond at the other two locations in skeleton (V) do not create any new structures.
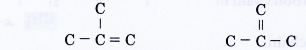
The above two structures are the same as skeleton (V). - Step 4: Place a hydrogen atom at each of the single bonds.
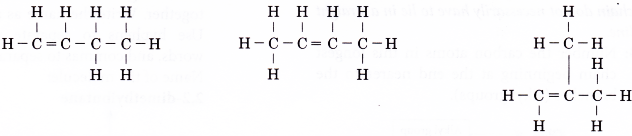
How to name isomers using IUPAC?
Naming isomers
- Isomers of alkanes and alkenes are also named according to the IUPAC rules. The name of a branched alkane or branched alkene consists of three component parts:
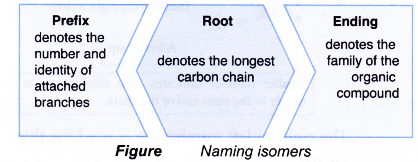
- The root and ending parts of the IUPAC name are obtained in the same way as in the naming of straight-chain alkanes and alkenes.
- For branched-chain alkanes or alkenes, prefixes are used to specify the identity, number and location of these branches or side chains.
- These branches are formally known as alkyl groups.
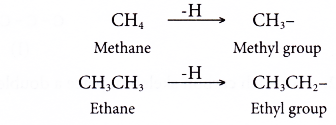
- An alkyl group is an alkane from which one hydrogen atom has been removed.
- Alkyl groups have the general formula CnH2n+1 where n = 1, 2, 3, …
- Alkyl groups are named by dropping -ane from the name of the corresponding alkane and replacing it with -yl. Two examples are shown below.
Naming isomers of alkanes example:
Name the alkane which has the structure shown below.
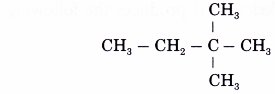
Solution:
- Step 1: Find the longest continuous carbon chain in the molecule.
- Step 2: Give the name for this longest chain.
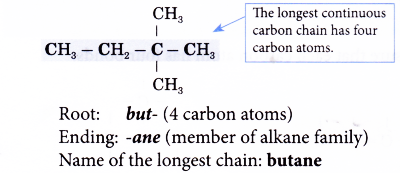
Note: The carbon atoms in the longest continuous chain do not necessarily have to lie in a straight line. - Step 3: Number the carbon atoms in this longest chain beginning at the end nearer to the branches (alkyl groups).
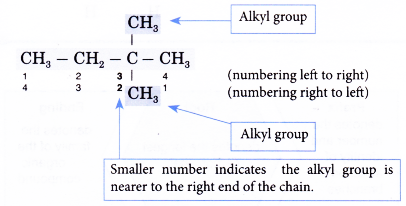
The right to left numbering is used for this alklane. - Step 4: Locate and name the attached alkyl groups. The position of each alkyl group is given the number of the carbon atom to which it is attached on the chain.
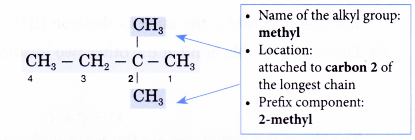
Note: If two or more of the same kind of alkyl groups are attached to the same chain, the number of alkyl groups is indicated by the prefixes di- (2), tri- (3), tetra- (4), and so on. In addition, the location of every identical group must be indicated by a number. - Step 5: Complete the name for the molecule by combining the three component parts together. Write the name as a single word. Use hyphens to separate numbers and words, and commas to separate numbers.
Name of the molecule:
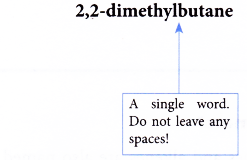
Table shows names and structural formulae of isomers of alkanes.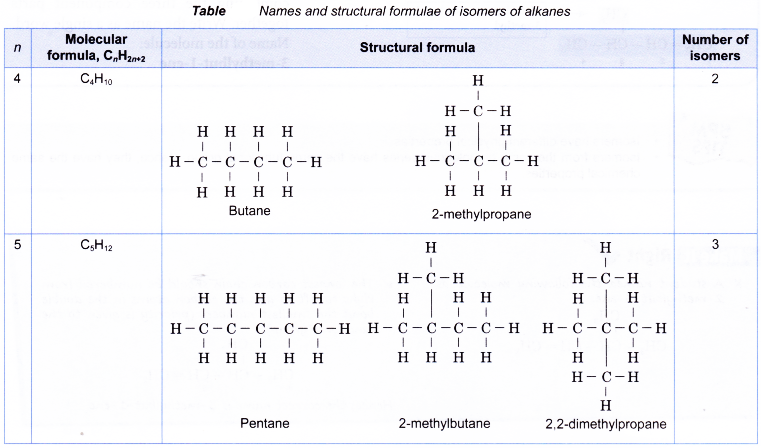
Naming isomers of alkenes example:
Name the alkene given below.
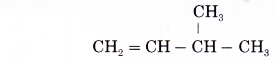
Solution:
- Step 1: Find the longest continuous carbon chain containing the double bond.
- Step 2: Give the name for this longest chain.
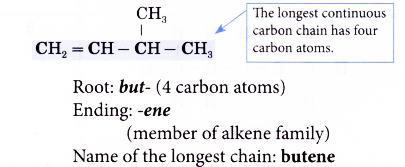
- Step 3: Number the carbon atoms in this longest chain beginning at the end nearer the double bond and not nearer to the first alkyl group. This will ensure the carbon atoms joined by the double bond have numbers as low as possible.

- Step 4: Locate the double bond by the number of the first carbon atom in the double bond. This number is placed in front of the family name.
Note: The position of the double bond needs to be indicated only for chains of four or more carbon atoms.
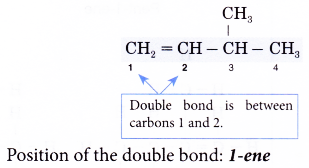
- Step 5: Locate and name the attached alkyl group.
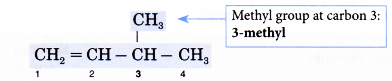
- Step 6: Complete the name for the molecule by combining the three component parts together. Write the name as a single word.
Name of the molecule: 3-methylbut-1 -ene
Table shows names and structural formulae of isomers of alkenes.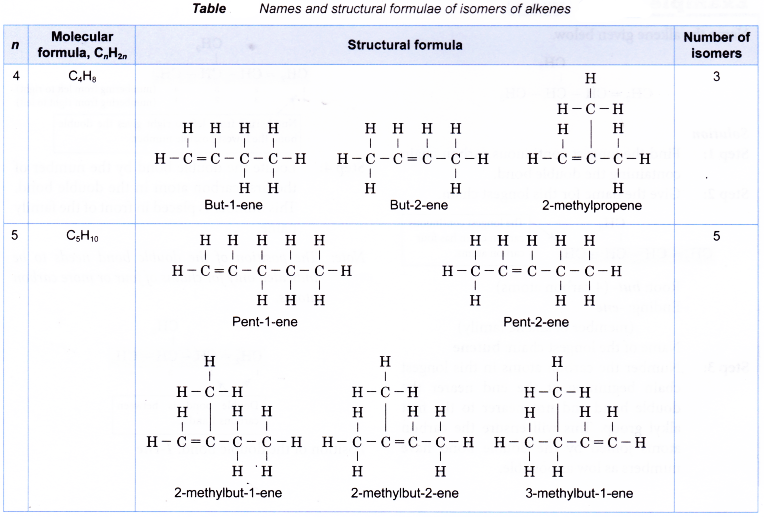
The post What is an isomerism? appeared first on A Plus Topper.
from A Plus Topper
via Learning Made Simple 360
*Note that these contents are Autoblogged from A Plus Topper and cannot be edited.
Join the conversation