What do you mean by transition metals?
What do you mean by transition metals?
Transition Elements
The position in the Periodic Table:
- Figure shows the positions of transition elements in the Periodic Table.

- Transition elements are elements from Group 3 to Group 12 of the Periodic Table.
- There are 10 elements in each serie arranged horizontally.
- The first series of transition elements is located in Period 4. It consists of the following elements.

Properties of transition elements
Table shows some properties of the first series of transition elements located in Period 4 of the Periodic Table.

Electronegativity
- Generally, transition elements have low electronegativity.
- However, the electronegativity increases slowly when going across the series from scandium to copper.
Physical properties
Physical properties of the transition elements do not change much when going across the period.
- Atomic radius (atomic size)
The atomic radii (atomic sizes) of the transition elements in the first series are almost the same. - Properties as metals
(i) All transition elements are metals.
(ii) Hence, all transition elements exhibit the following physical properties of metals.- They are solids with shiny surfaces.
- They are ductile and malleable.
- They have high tensile strengths.
- They have high melting and boiling points.
- They have high densities.
- They are good conductors of heat and electricity.
- Tensile strength is the ability of metals to maintain their shape without breaking when a force is applied to it.
Ductile is the ability of metals to be stretched into wires without cracking or breaking them.
Malleable is the ability of metals to be bent into a new shape.
People also ask
- What is the periodic table of the elements?
- Physical and Chemical Properties of Group 1 Elements
- Physical and Chemical Properties of Group 18 Elements
- Physical and Chemical Properties of Group 17 Elements
- What is the Need for Classification of Elements?
- Modern Periodic Table and Its Significance
- How did Mendeleev Arrange the Periodic Table?
- Periodic Trends in Properties of Elements
- What is Valency and Atomic Radius?
- What are Metallic and Nonmetallic Properties?
Uses of transition elements
Transition elements are widely used in various fields. For example:
- Iron is widely used in steel for the construction of bridges and buildings, and in the making of cutlery, engineering equipments and vehicles.
- Copper is used as electric wires or cables because it is malleable and ductile and is a very good conductor of electricity.
Although zinc is located in the first series of the transition elements, it does not exhibit the characteristics of transition elements. Hence, zinc is not a transition element.
Special characteristics of transition elements
Transition elements are metals that exhibit four special characteristics which are not possessed by other metals. These four characteristics are:
1. Form coloured ions or compounds
Compounds of transition elements are coloured in the solid state or aqueous solution.
Table shows the colours of some compounds of transition elements in the solid state.
| Compound of transition element | Colour |
| Cobalt chloride crystal | Pink |
| Copper(II) sulphate crystal | Blue |
| Iron(II) sulphate crystal | Pale green |
| Iron(III) sulphate crystal | Brown |
The aqueous solutions of these compounds are also coloured because of the existence of ions of transition elements.
Table shows the colours of some ions ions of transition elements in aqueous solutions.
| Ions of transition element | Formula of the ion | Colour of ion in aqueous solution |
| Copper(II) ion | Cu2+(aq) | Blue |
| Iron(II) ion | Fe2+(aq) | Pale green |
| Iron(III) ion | Fe3+(aq) | Yellow / yellowish- brown / brown |
| Cobalt(II) ion | Co2+(aq) | Pink |
| Nickel(II) ion | Ni2+(aq) | Green |
| Chromium(III) ion | Cr3+(aq) | Green |
| Manganese(II) ion | Mn2+(aq) | Light pink |
| Manganate(VII) ion | MnO4–(aq) | Purple |
| Chromate(VI) ion | CrO42-(aq) | Yellow |
| Dichromate(VI) ion | Cr2O72-(aq) | Orange |
Reactions of aqueous solutions of transition element compounds with sodium hydroxide solution and ammonia solution
- Aqueous solutions of transition element compounds are coloured.
- These aqueous solutions can react with sodium hydroxide solution and ammonia solution to form coloured precipitates of metal hydroxides. These precipitates may be soluble/insoluble in excess sodium hydroxide solution and ammonia solution.
- The precipitates of the metal hydroxides formed are coloured because these are compounds of transition elements.
Table shows some examples of these reactions.

Uses of coloured transition element compounds
In our daily life, compounds of transition elements are added to paints and glass to make them coloured.
For example:
- Green glass is made by adding a mixture of chromium(III) oxide and copper(II) oxide to the glass.
- Yellow paint is made by adding barium chromate(VI) to the paint.
Existence of naturally occurring substances containing transition element compounds
- Precious stones (gemstones) are naturally occurring substances that exist in different colours.
- Precious stones are coloured naturally because of the existence of the compounds of transition elements in them.
- Precious stones are usually used as ornaments and decorative pieces.
- Table shows the colours and the types of compounds of transition elements that exist in a few precious stones
Precious stone
Colour Transition elements that exist in the form of compounds Emerald Green Nickel, iron Ruby Red Chromium Sapphire Blue Iron, titanium Amethyst Purple Iron Agate Brownish-red Manganese, iron
2. Exhibit different oxidation numbers in compounds
- Transition elements exhibit different oxidation numbers in their compounds.
- Oxidation number measures the charge carried by an element in its compounds. It has a positive or negative sign.
- Table shows the oxidation numbers of some transition elements in their compounds.
Transition element Oxidation number in compounds Iron +2, +3 Nickel +2, +3 Copper +1, +2 Manganese +2, +3, +4, +6, +7 Chromium +2, +3, +6
Naming of compounds
- In the naming of compounds of transition elements with more than one oxidation number, Roman numerals that represent the oxidation numbers of that transition element must be inserted in the names of those compounds according to the IUPAC system.
- For example:
FeCl2 is named as iron(II) chloride because the oxidation number of iron is +2. FeCl3 is named as iron(III) chloride because the oxidation number of iron is +3.
3. Form complex ions
- Transition elements can form complex ions.
- A complex ion is a bigger-sized polyatomic ion formed when a fixed number of small molecules or ions (known as ligands) are bonded to a central transition metal ion.
- An example of a complex ion formed by a transition element is tetraamminecopper(II) ion, [Cu(NH3)4]2+. This complex ion consists of four ammonia molecules, NH3 (ligands) bonded to the central copper(II) ion, Cu2+.
Cu2+ + 4NH3 → [Cu(NH3)4]2+ - Other examples of complex ions are:
- Hexacyanoferrate(II) ion, [Fe(CN)6]2-
- Hexacyanoferrate(III) ion, [Fe(CN)6]3-
- Hexaaquocobalt(II) ion, [Co(H,0)6]2+
- Hexaamminechromium(III) ion, [Cr(NH3)6]3+
4. Act as catalysts
How do transition elements act as catalysts?
Transition elements or their compounds can act as catalysts in certain reactions.
Catalysed chemical reactions
- Catalysts are used in chemical reactions to increase the rate of reaction.
- The following are a few laboratory reactions that use catalysts.
- Nickel acts as a catalyst in the hydrogenation of alkene to form the corresponding alkane.
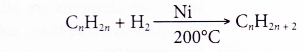
- Copper(II) sulphate acts as a catalyst in the reaction of zinc with dilute sulphuric acid to liberate hydrogen gas.
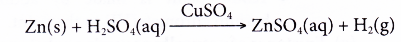
- Manganese(IV) oxide is used as a catalyst in the decomposition of hydrogen peroxide to liberate oxygen gas.
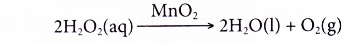
- Nickel acts as a catalyst in the hydrogenation of alkene to form the corresponding alkane.
Catalysed industrial processes
- Catalysts are used in industrial processes to speed up reactions.
- A few industrial processes that use transition elements or their compounds as catalysts are:
Haber process:
Iron is used as a catalyst in the Haber process to manufacture ammonia from the reaction of nitrogen gas with hydrogen gas at 450°C to 550°C and a pressure of 200 to 300 atmosphere.
![]()
Contact process:
Vanadium (V) oxide is used as a catalyst in the Contact process to manufacture sulphuric acid. Vanadium(V) oxide catalyses the reaction between sulphur dioxide and oxygen (air) to produce sulphur trioxide at about 500°C and a pressure of one atmosphere.
![]()
Ostwald process:
Platinum is used as a catalyst in the Ostwald process to manufacture nitric acid. Platinum catalyses the reaction between ammonia and oxygen (air) to produce nitrogen monoxide and water at about 850°C and a pressure of 2 to 5 atmospheres.
![]()
Hydrogenation of vegetable oil:
Nickel is used as a catalyst in the hydrogenation of vegetable oil to manufacture margarine. Nickel catalyses the reaction between vegetable oil and hydrogen gas at 200°C.
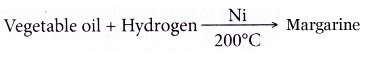
The post What do you mean by transition metals? appeared first on A Plus Topper.
from A Plus Topper
via Learning Made Simple 360
*Note that these contents are Autoblogged from A Plus Topper and cannot be edited.
Join the conversation