How are carboxylic acids formed?
How are carboxylic acids formed?
What is the general formula of a carboxylic acid?
Carboxylic Acids
-
- Carboxylic acids is another family of non-hydrocarbons, containing the elements carbon, hydrogen and oxygen.
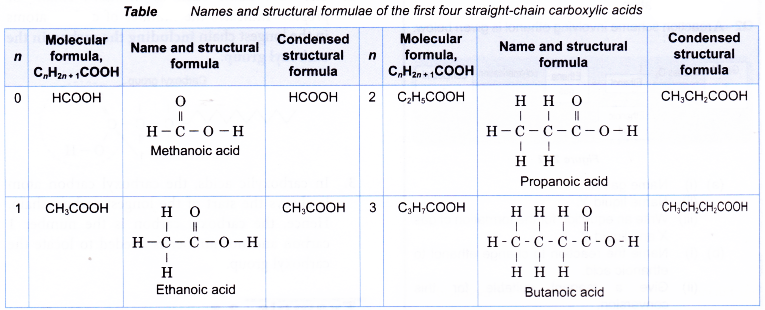
- The general formula of the carboxylic acid family is CnH2n+1COOH, where n = 0, 1, 2, 3,…
- The –COOH group is the functional group of the carboxylic acids. It is called the carboxyl group.
- The structure of the functional group is written as
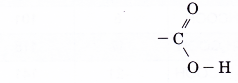
Note that one oxygen atom is bonded by a double bond and the other oxygen atom is bonded by a single bond. The carboxyl group determines the reactions of the carboxylic acids.
How do you name a carboxylic acid?
Naming carboxylic acids
- Straight-chain carboxylic acids are named by dropping the -e from the name of the corresponding alkane and replacing it with -oic acid.
- The root name such as methan-, ethan- or propan- indicates the number of carbon atoms in the longest chain including the carbon in the carboxyl group.
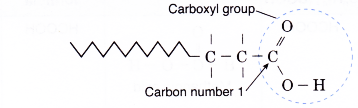
- In carboxylic acids, the carboxyl carbon atom is always the start of the longest carbon chain. Hence, the carboxyl carbon is the number 1 carbon and no number is needed to locate the carboxyl group.
Naming carboxylic acids example:
Name the carboxylic acid shown below.
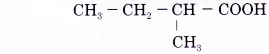
Solution:
Step 1: Find the longest continuous carbon chain containing the carboxyl group.
Step 2: Name this longest chain by replacing the ending -e of the corresponding alkane with -oic acid.
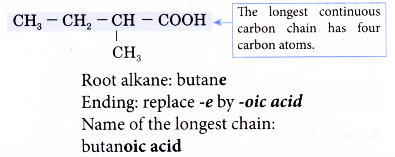
Step 3: Number the carbon atoms in this longest chain beginning at the carboxyl group.

Step 4: Locate and name the attached alkyl group.
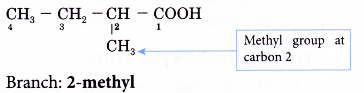
Step 5: Complete the name for the carboxylic acid molecule by combining the two component parts together.
Name of the molecule: 2-methylbutanoic acid.
People also ask
- What are carbon compounds?
- Chemical Properties of Carbon Compounds
- How are alkanes formed?
- What is an alkene in chemistry?
- What is an isomerism?
- What is alcohol and how is it made?
- How esters are formed?
- What are fats and oils?
- How palm oil is extracted?
- Order in Homologous Series
- What is the monomer of natural rubber?
- Which acid is used for coagulating rubber from latex?
- Classification of Hydrocarbons
- What is the homologous series of hydrocarbons?
- Properties and Uses of Ethanol
- Properties and Uses of Ethanoic Acid
Physical properties of carboxylic acids
- Carboxylic acids with low molecular mass are colourless liquids at room temperature. Larger carboxylic acids that have ten or more carbon atoms are wax-like solids.
- The simple carboxylic acids have sharp or unpleasant odours.
- Boiling points of carboxylic acids are higher than that of alkanes with the same number of carbon atoms. More energy is required to overcome the stronger forces of attraction between the larger carboxylic acid molecules.
- Within the carboxylic acid family, the boiling points increase with increasing number of carbon atoms per molecule. Table shows the melting and boiling points of the first four carboxylic acids.
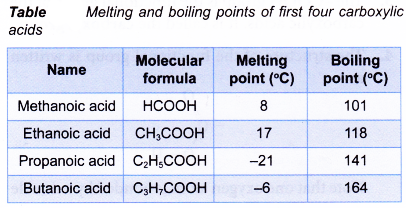
- Carboxylic acids with four carbon atoms or fewer are very soluble in water. The high solubility is due to water molecules being strongly attracted to the carboxyl group.
- However, the solubility decreases with increasing number of carbon atoms per molecule. The longer carboxylic acid molecule tends to be alkane-like and becomes insoluble in water.
How do you make Ethanoic acid?
Making ethanoic acid
- The most common laboratory preparation of a carboxylic acid is by the oxidation of an alcohol.
- The oxidation of ethanol is used to prepare ethanoic acid.
- This is carried out by refluxing ethanol with an oxidising agent such as acidified potassium dichromate(VI) solution or acidified potassium manganate(VII) solution.
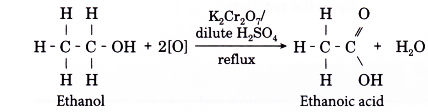
- The reaction is carried out using the set-up of the apparatus as shown below.
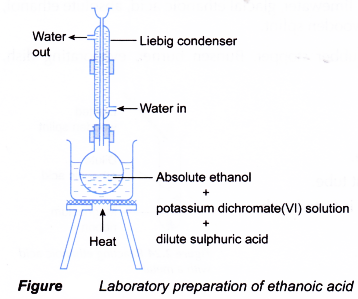
The flask is fitted with an upright condenser to prevent the loss of a volatile liquid by vaporisation. This method of retaining a volatile liquid during heating is called refluxing. - During the oxidation of ethanol, the orange dichromate(VI) ions turn to green.
- The ethanoic acid formed is removed by fractional distillation.
Physical properties of ethanoic acid
- Ethanoic acid is a colourless liquid at room conditions. Pure ethanoic acid is known as glacial ethanoic acid.
- It has a sour smell like vinegar.
- Ethanoic acid is very soluble in water.
Chemical properties of ethanoic acid
- The chemical reactions of the carboxylic acids can be understood by studying the reactions of ethanoic acid.
- The reactive site in the carboxylic acids is the carboxyl group, -COOH. This is the group that controls the chemical reactions of the carboxylic acids.
- Acid properties
(a) Ethanoic acid is a weak monoprotic acid. It ionises partially in water to produce a low concentration of hydrogen ions.
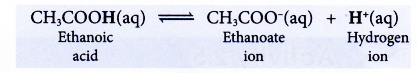
(b) Only the hydrogen atom in the carboxyl group, -COOH can ionise in water to produce hydrogen ion.
(c) Ethanoic acid turns moist blue litmus paper red and reacts just like any typical acid. Unlike strong mineral acids such as sulphuric acid and hydrochloric acid, the weak ethanoic acid reacts slowly with metals, bases and carbonates. - Reactions with metals
(a) Dilute ethanoic acid reacts with reactive metals to produce a salt and hydrogen. Copper and metals below it in the reactivity series cannot react with ethanoic acid.
(b) The following equation shows the reaction between zinc and ethanoic acid.
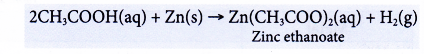
A colourless solution of zinc ethanoate is formed. - Reactions with bases
(a) Dilute ethanoic acid neutralises alkalis such as sodium hydroxide solution to give a salt and water. The salt formed is sodium ethanoate.
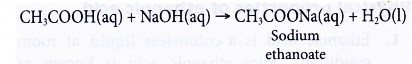
(b) Black copper(II) oxide power dissolves in hot dilute ethanoic acid to form a blue solution of copper(II) ethanoate and water.
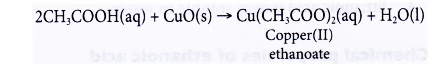
- Reactions with carbonates
(a) Acids react with metal carbonates to produce a salt, carbon dioxide and water.
(b) Ethanoic acid behaves the same way. Reaction between ethanoic acid and calcium carbonate produces a colourless solution of calcium ethanoate and effervescence of carbon dioxide.
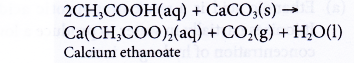
- Reactions with alcohols
(a) Carboxylic acid reacts with alcohol to form an ester and water. This reaction produces a sweet-smelling compound called an ester. The reaction process is called esterification.
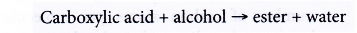
(b) When a mixture of ethanoic acid and butan-l-ol with a few drops of concentrated sulphuric acid is heated, an ester called butyl ethanoate is formed.
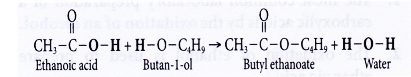
(c) Butyl ethanoate is a colourless sweet-smelling liquid. It forms an oily layer which floats on water.
(d) Concentrated sulphuric acid is a catalyst. It helps to speed up the reaction and is not used up in the reaction.
Chemical properties of ethanoic acid experiment
Aim: To investigate the chemical properties of ethanoic acid.
Materials: 1.0 mol dm-3 ethanoic acid, 1.0 mol dm-3 sodium hydroxide solution, magnesium ribbon, copper(II) oxide, calcium carbonate, iron(III) carbonate, zinc, limewater, glacial ethanoic acid, absolute ethanol, propan-1-ol, concentrated sulphuric acid, water, wooden splint.
Apparatus: Test tube, stopper with delivery tube, spatula, rubber stopper, Bunsen burner, evaporating dish, dropper, boiling tube, beaker, test tube holder.
A. Reaction of ethanoic acid with a metal
Procedure:
- About 2 cm3 of dilute ethanoic acid is placed in a test tube.
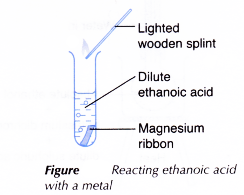
- A piece of clean magnesium ribbon is dropped into the test tube.
- The test tube is closed to collect the gas liberated. The gas is tested with a lighted wooden splint.
- Steps 1 to 3 are repeated using zinc to replace magnesium.
Observations:
| Metal | Observation |
| Magnesium |
|
| Zinc |
|
Discussion:
- Magnesium reacts with dilute ethanoic acid to produce magnesium ethanoate and hydrogen gas.
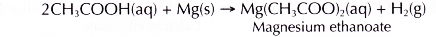
- Zinc reacts with dilute ethanoic acid to produce zinc ethanoate and hydrogen gas.
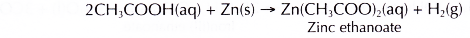
B. Reaction of ethanoic acid with a base
Procedure:
- About 2 cm3 of dilute ethanoic acid is placed in a test tube.
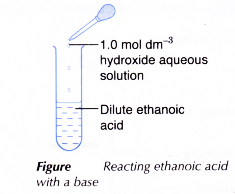
- About 2 cm3 of 1.0 mol dm-3 sodium hydroxide solution is added to the acid and the mixture is shaken.
- The mixture is poured into an evaporating dish and heated to dryness.
- The solid residue is examined.
- Steps 1 to 4 are repeated using one spatulaful of copper(II) oxide powder to replace 2 cm3 of sodium hydroxide solution.
Observations:
| Base | Observation |
| Sodium hydroxide solution |
|
| Copper(II) oxide |
|
Discussion:
- Sodium hydroxide solution neutralises ethanoic acid to form a salt, which is sodium ethanoate and water.
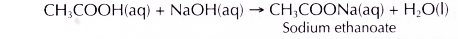
- Ethanoic acid dissolves copper(II) oxide to produce copper(II) ethanoate and water.
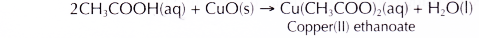
The copper(II) ions cause the solution to become blue.
C. Reaction of ethanoic acid with a metal carbonate
Procedure:
- About 2 cm3 of dilute ethanoic acid is placed in a test tube.
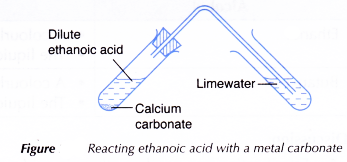
- One spatulaful of calcium carbonate is added to the acid.
- The test tube is closed with a stopper fitted with a delivery tube dipping into limewater. Any change to the limewater is noted.
- Steps 1 to 3 are repeated using iron(III) carbonate to replace calcium carbonate.
Observation:
| Metal carbonate | Observation |
| Calcium carbonate |
|
| Iron(III) carbonate |
|
Discussion:
- Calcium carbonate reacts with dilute ethanoic acid to produce calcium ethanoate, water and carbon dioxide.
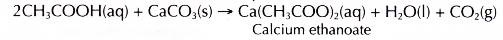
- Iron(III) carbonate reacts with ethanoic acid to produce iron(III) ethanoate, water and carbon dioxide.
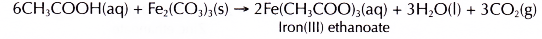
The iron(III) ions make the salt solution brown.
D. Reaction of ethanoic acid with alcohol
Procedure:
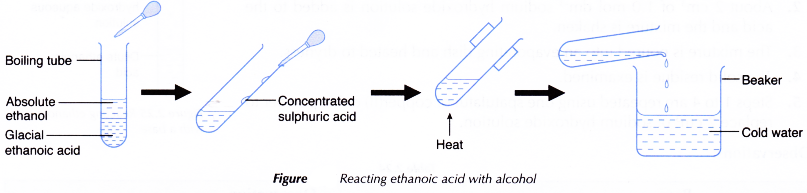
- About 2 cm3 of glacial ethanoic acid is poured into a boiling tube.
- About 2 cm3 of absolute ethanol is added to the acid.
- The boiling tube is shaken to mix the liquids well.
- A dropper is used to add about 1 cm3 of concentrated sulphuric acid to the mixture. The boiling tube is shaken well.
- The mixture is carefully heated over a small flame. The mixture is allowed to boil gently for about 2 to 3 minutes.
- A beaker is half-filled with some water.
- The contents of the boiling tube are poured into the beaker.
- Any change that occurs is observed.Steps 1 to 7 are repeated using butan-1-ol to replace absolute ethanol.
Observations:
| Alcohol | Observation |
| Ethanol |
|
| Butan-1 -ol |
|
Discussion:
- Esterification occurs when ethanoic acid reacts with an alcohol to form an ester and water.
- Ethanoic acid reacts with ethanol to produce ethyl ethanoate.
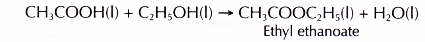
- Ethanoic acid reacts with butan-1-ol to produce butyl ethanoate.
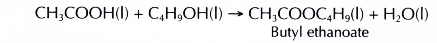
- Concentrated sulphuric acid functions as a catalyst.
Conclusion:
Ethanoic acid reacts with metal, base, metal carbonate and alcohol.
Chemical reactions of other carboxylic acids
- All members of the carboxylic acid homologous series have the same functional group as ethanoic acid, which is the carboxyl group, -COOH. Therefore, the other carboxylic acids should undergo the same chemical reactions as ethanoic acid.
- Carboxylic acids react with reactive metals.
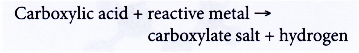
Example:
Propanoic acid reacts with sodium to form sodium propanoate and hydrogen.
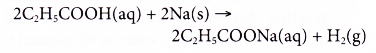
- Carboxylic acids react with bases.
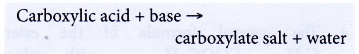
Example:
Butanoic acid reacts with magnesium oxide to form magnesium butanoate and water.
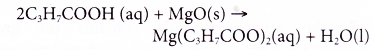
- Carboxylic acids react with metal carbonates.

Example:
Propanoic acid reacts with calcium carbonate to form calcium propanoate, water and carbon dioxide.
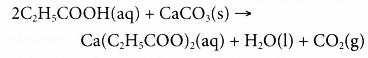
- Carboxylic acids react with alcohols.
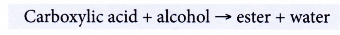
Example:
Butanoic acid reacts with ethanol to form ethyl butanoate and water.
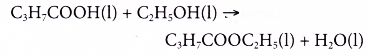
What is the use of carboxylic acid?
Uses of carboxylic acids
- Ethanoic acid (commonly called acetic acid) is a major industrial chemical. Large quantities of it are manufactured for its use in food as flavouring and as a preservative.
- Vinegar is a solution containing 5% ethanoic acid.
- Ethanoic acid is also used with other chemicals to make drugs, dyes, paints, insecticides and plastics.
- Ethanoic acid can be converted into esters for the use as solvents.
- In the rubber industry, methanoic acid (formic acid) is used to coagulate latex.
- Fatty acids are long-chain carboxylic acids used in making soaps.
- Carboxylic acids are used in the manufacture of polyesters and polyamides, which are fibres used in the textile industry.
- Benzoic acid is a preservative in foods.
Reaction map for ethanoic acid
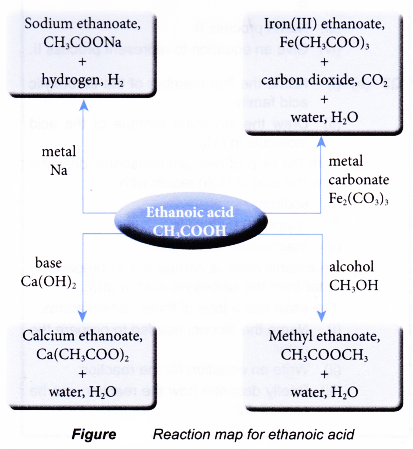
The post How are carboxylic acids formed? appeared first on A Plus Topper.
from A Plus Topper
via Learning Made Simple 360
*Note that these contents are Autoblogged from A Plus Topper and cannot be edited.
Join the conversation